48+ The Mass Number Is Used To Calculate The Number Of
Calculating numbers of subatomic particles The symbol for an atom can be written to show its mass number at the top and its atomic number at the bottom. 6 The mass number is used to calculate the number of protons and neutrons in one.
Papers Past Parliamentary Papers Appendix To The Journals Of The House Of Representatives 1936 Session I Public Works Statement By The Hon R Semple
Mass number is the sum of the number of protons and the number of neutrons in the atom.

. Given Atomic number 13 We know that Z Atomic Number Number of protons Therefore number of proton13 We know that Mass. The mass number is used to calculate the number of _____ - 10328111. The mass number is used to calculate the number of _____.
6 the mass number is used to calculate the number of. I have to write a bunch of. Mass Number calculator uses Mass Number Number of ProtonsNumber of Neutrons to calculate the Mass Number The Mass number is the sum of the number of protons and.
Knowing the number of neutrons and the atomic number or the number of protons or electrons of an atom we can determine the mass number of an element. The mass number is used to calculate the number of _____. School Tallwood High School.
To calculate the numbers of. There cannot be another element with the atomic number 19. The mass number is used to calculate the number of protons and neutrons in one atom of an element.
How do you calculate a mass number. 6 The mass number is used to calculate the number of nucleonsneutrons in one from HISTORY 110 at Casa Grande High. While mass is defined by F ma in situations where density and volume of the object are known mass is also commonly calculated using the following equation as in the calculator provided.
The atomic number tells you the number of protons in the nucleus of the atom. Mass Number Atomic Number Number of Neutrons. Mass of 1 atom mass of a mole of atoms 6022 x 10 23 mass of 1 C atom 1201 g 6022 x 10 23 C atoms mass of 1 C atom 1994 x 10 -23 g Answer The mass of a.
Mass Number Atomic Number. Home Chemistry homework help. Mass number is the sum of the number of protons and the number of neutrons in the atom.
As per the periodic table relative atomic masses of Carbon C 12 Oxygen O 16 and Calcium Ca 40. Mass of 05 moles of. The atomic number is the number of protons in the nucleus of an.
Mass of 1 mole of. The Atomic mass is used to calculate the number of Neutrons in an atom. Next on the button we have the average atomic mass of.
Calculate the number of protons and neutrons. Calculate Mass of cell. So potassium has 19 protons and this is true for all its isotopes.
Atomic number from the atomic mass. Every atom is composed of Protons and Neutrons forming a tight compact nucleus orbited by electrons. This mole calculator is able.
Mass number protons neutrons plss i rlly need itt.

Practice Ws 3

20msft Levels Number Of Shuttles Per Level And Time Seconds S And Download Scientific Diagram
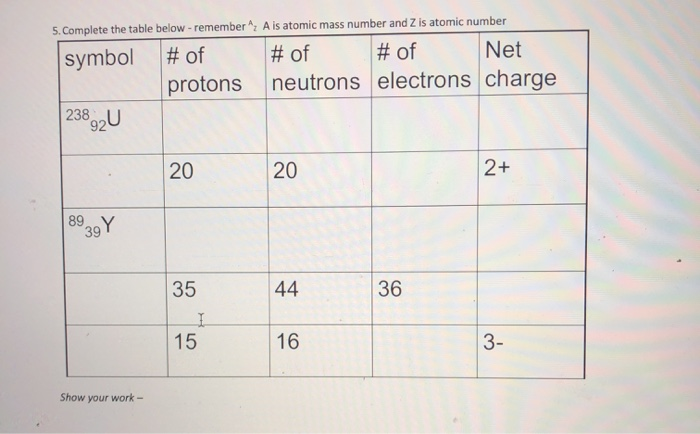
Solved A Is Atomic Mass Number And Z Is Atomic Number 5 Chegg Com

Solved 7 An Atom Has A Mass Number Of 18 And An Atomic Chegg Com

Mass Number Chemistry For Non Majors Course Hero

Distribution Coefficient Model For Zirconium And Technetium Extraction From Nitric Acid Solution Industrial Engineering Chemistry Research
Metabolic Excretion Associated With Nutrient Growth Dysregulation Promotes The Rapid Evolution Of An Overt Metabolic Defect Plos Biology

Seven Coordinated Molecular Ruthenium Water Oxidation Catalysts A Coordination Chemistry Journey Chemical Reviews

Atomic Number Mass Number Examples Solutions Videos

Nuclear Charge
Effect Of Weight Of Larvae X Axis And Ambient Temperature Boxes On Download Scientific Diagram
Production Layouts Anno 1800 Wiki Fandom
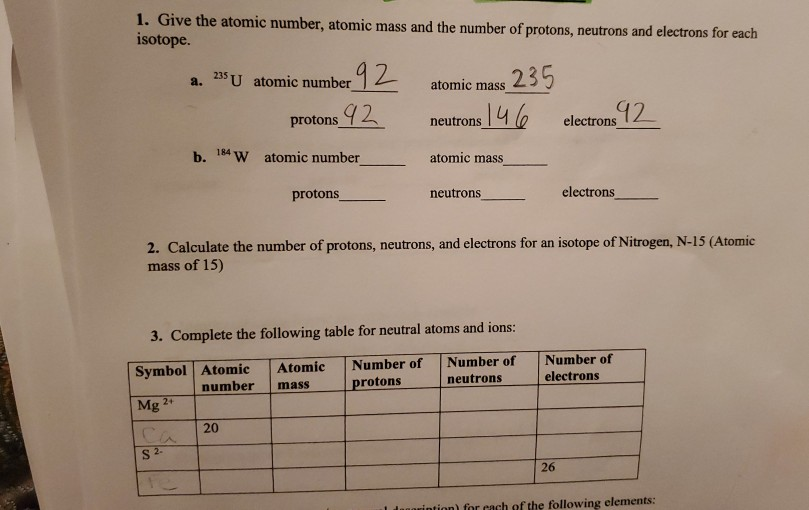
Solved 1 Give The Atomic Number Atomic Mass And The Number Chegg Com
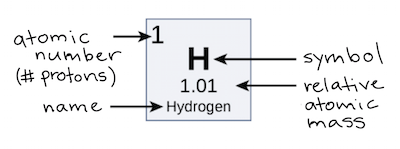
Atomic Number Atomic Mass And Isotopes Article Khan Academy
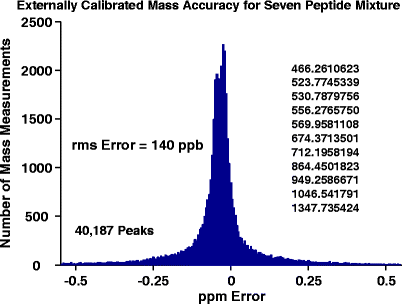
21 Tesla Fourier Transform Ion Cyclotron Resonance Mass Spectrometer A National Resource For Ultrahigh Resolution Mass Analysis Springerlink

Nuclear Energy Examples Pdf Examples

Scientific Recommendations For Strength And Hypertrophy Training From 150 Studies Sci Fit